New NECC Research Certificate Addresses Industry Shortage

NECC Professor Emily Gonzalez, coordinator of the Clinical Research Coordinator Certificate Program (left) and Terry Stubbs, founder and CEO of ActiveMed, collaborated on creating the new program.
When Northern Essex Community College alumnae Terry Stubbs, founder of ActivMed Practices & Research, Inc., and Cynthia Owens, a director at Affiliated Monitors, Inc. had trouble finding qualified clinical research coordinators, they turned to the one institution they knew could help – their alma mater.
Stubbs and Owens collaborated with Northern Essex to create a certificate program that would prepare individuals who already work in the sciences or health care fields to add to their skill set for immediate employment in the clinical research field. The new clinical research coordinator certificate is offered through iHealth @NECC and launches in the fall 2017 semester. All coursework is online. Students must participate in a face-to-face clinical research practicum course for a minimum of 180-hours. ActivMed is one of those sites,
Stubbs, A Hampton Falls, NH resident, who launched ActivMed 22 years ago, now oversees four locations – Methuen, Lawrence, Beverly, and Portsmouth. She said for the last three years there has been a national shortage of clinical research coordinators.
Owens, of Amesbury is co-founder of “Asentral Institutional Review Board. She is delighted with the creation of the program. “This program should appeal to a broader audience than any other currently available,” she said. “Whether you are a scientist or not, you can now be trained on the basic tenets of clinical trials and be a part of advancements in providing medical solutions.”
“Terry and Cynthia were adamant that the industry really needs skilled workers and Northern Essex should be on the forefront of providing those workers,” said Kim Burns, NECC’s dean of academic innovations and interim dean of professional development.
NECC’s Academic and Student Affairs Division worked with focus groups comprised of administrators from Boston hospitals and medical companies to create a three semester, 10-month certificate program that addresses the needs of the local economy while offering a solid entry-level education to the expanding field of clinical research to individuals already holding degrees in health care or science fields.
The first cohort will begin in January, 2017. Future cohorts will begin in the fall.
“We are really excited about this program,” said Emily González, coordinator of the Clinical Research Coordinator Certificate Program. “This is a very interesting and exciting field to get into. No two research studies are the same. It’s ideal for anyone who loves details and analysis.”
Stubbs agrees, “In the past it has taken two years for us to bring someone in who already has a science degree and train them in clinical research. Our hires have had the medical knowledge, but not the research knowledge. After they earn a certificate we will both be able to hit the ground running.”
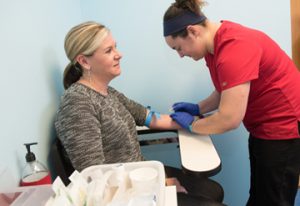
Clinical research coordinators help administer clinical research trials by collecting data from study
participants, monitoring clinical trial procedures, maintaining research logs, and ensuring proper research guidelines are followed.
According to Indeed.Com, a leading employment site, the average income in the Boston area for a clinical research coordinator is more than $45,000.
The NECC clinical research coordinator certificate program is designed for individuals who already hold an associate, bachelor’s, or even master’s degree in a health care or science field, but would like to enter the world of clinical trials where the information is used to develop new medications, medical devices, or diagnostic tools. González, the program coordinator, may also give permission to individuals who have worked for a number of years in health care with only a diploma.
“We will be credentialing individuals already in the industry” Burns says.
Prior to enrolling in the certificate program individuals must have completed Anatomy and Physiology I and II with a minimum grade of “C” and have earned as associate or bachelor’s degree in a related health or science field with a GPA of 2.0 or higher.
“It’s a really exciting field because it’s constantly changing. Once you start working in research you get addicted,” Stubbs said. “I’m ecstatic about this program. There really is a national shortage. Finish this program and you will definitely get hired at a research company.”
“Something that began as a conversation between two colleagues identifying a significant need in our industry, has evolved in a relatively short time and now has a robust solution,” said Owens.
General iHealth information sessions will be held at NECC on Wednesdays at 78 Amesbury St. LA-105, in Lawrence on the following dates: November 16, 4:30 p.m.; November 30, 4:30 p.m.; December 14, 10 a.m.; January 4, at 4:30 p.m.; January 18, 2017, 4:30 p.m.; and Feb 1, 2017, 10 a.m.
For more information on the program please contact iHealth advisors Linda Comeau at 978-738-7610 or lcomeau@necc.mass.edu or Cristina Nuncio at 978-7387709 or cnuncio@necc.mass.edu or visit the website .